Institut Charles Sadron News
Publié le 13/02/2026 par Kekicheff Patrick
Researchers from Grenoble Alpes University and Charles Sadron Institute of the C.N.R.S. (Strasbourg) publish in PNAS the resolution of a controversy more than ten years old. Electrostatic screening in ionic liquids — the ability of ions to neutralize a charge — is actually short-range, unlike previous conflicting experimental reports of abnormally long-range interactions.
A scientific enigma solved
Ionic liquids — organic molten salts that are liquid at room temperature — have attracted increasing interest for their remarkable properties. Their non-flammability, non-volatility, thermal and chemical stability, high ion conductivity, enable numerous applications in bulk (catalysis, electrochemistry) and at the interfaces (lubrication, electrowetting, energy storage, batteries). But over what distance do the electrical charges influence each other? Their range determines all applications. For a dozen years, experiments reported abnormally large screening lengths far exceeding theoretical predictions and contradicting numerical simulations, a phenomenon that was called underscreening. But the new results show that the key lies in the measurement conditions and the crucial importance of approaching thermodynamic equilibrium.
Praise of slowness
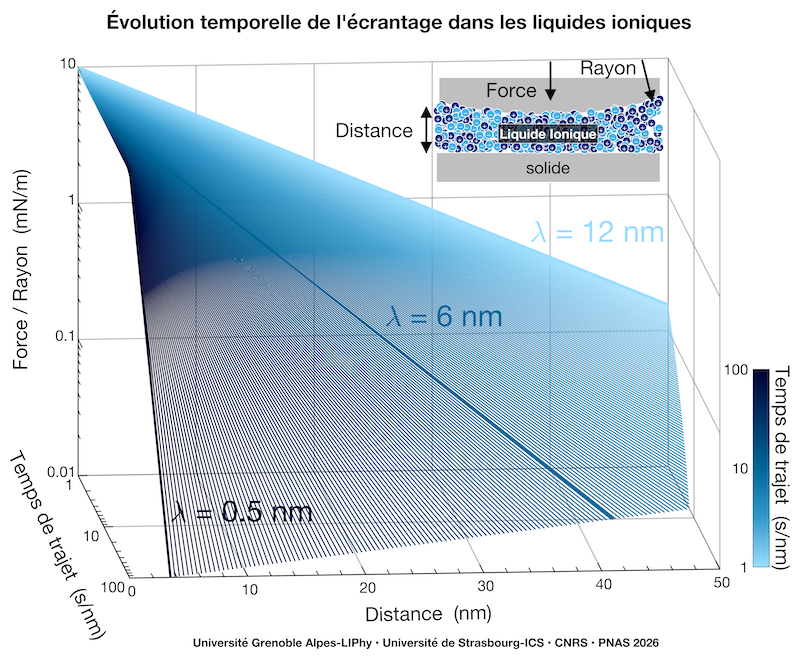
The researchers used two complementary Surface Force Apparatuses (SFAs) to measure interaction profiles as a function of the distance between two surfaces confining ionic liquids in a variety of quasi-static and dynamic conditions and in different geometries. They achieved the technical feat of reducing the speed of movement of the confining walls to 9 picometer per second (at this speed, it would take 4 millennia to travel one meter). The results reveal that an apparent screening length emerges, but its value strongly depends on the time given to the ionic liquid to organize itself. At fast velocities (one second to travel a nanometer), the interactions seem to extend far, to more than ten nanometers. But by slowing down by two orders of magnitude (100 seconds per nanometer), this range shrinks and converges to 0.5 nanometers — the typical size of an ion.
An excessively slow relaxation dynamic
The relaxation towards equilibrium extends over two orders of magnitude in time, reminiscent of aging-like phenomena observed in a variety of systems, including glasses, thin polymer films, granular media, historical heritage paintings. In these systems with complex and hierarchical energy landscapes, the material slowly explores multiple configurations before a balance through dynamical heterogeneities is reached. This intrinsic slowness explains why rapid measurements, not allowing the electrolyte to find its stable configuration, gave misleading results.
Practical implications
These results clarify the fundamental parameter that is the screening length at the core of the optimization of batteries and supercapacitors based on ionic liquids. They also impact industrial lubrication and the design of nanofluidic devices. The established rigorous methodology paves the way for the study of other concentrated electrolytes and systems with complex multiscale spatial and slow time relaxation.
Reference
Title: Short-range electrostatic screening in ionic liquids as inferred by direct force measurements
Autors : Benjamin Cross¹*, Léo Garcia¹, Elisabeth Charlaix¹, Patrick Kékicheff²*
Address : 1. Université Grenoble Alpes, CNRS, LIPhy, Grenoble
2. Institut Charles Sadron, Université de Strasbourg, C.N.R.S. UPR22, Strasbourg
Publication: Proceedings of the National Academy of Sciences (PNAS) 123 (No. 7) e2517939123 (2026)
DOI: https://doi.org/10.1073/pnas.2517939123
Financing: Agence Nationale de la Recherche (No. ANR-19-CE30-0012) & Plate-forme MICASOL
Contacts (*)
Benjamin Cross — Université Grenoble Alpes, LIPhy, benjamin.cross@univ-grenoble-alpes.fr
Patrick Kékicheff — Institut Charles Sadron, C.N.R.S., patrick.kekicheff@ics-cnrs.unistra.fr